stevep
Elder Statesman
  
Posts: 1,073
|
Post by stevep on Apr 16, 2020 18:17:19 GMT
Hi Steve. Lovely job!! I'm pleased to see you will be removing the zinc coating. While zinc plating is good at preventing rust, it also prevents paint from adhering well. Even etch primer doesn't help much. There are etch primers made specifically for use on galvanised steel, but even they don't adhere as well as on plain steel or brass. The only way to guarantee paint sticks to galvanised is to actually 'weather' it outside for 24 months. Not really a suitable idea for model engineering projects!! Bob. You should see how long some of my engines took to build! |
|
|
|
Post by springcrocus on Apr 16, 2020 18:27:09 GMT
I discovered by accident that citric acid pickle will dissolve the zinc. However, because my pickle has a lot of dissolved copper in it, I later found on the internet that the copper ions accelerates the process almost tenfold. They came out of the tank just over an hour later and are completely free of zinc.
Regards, Steve
|
|
|
|
Post by steamer5 on Apr 17, 2020 0:50:28 GMT
Hey Steve,
By chance do you get a copper coat on the steel?
Nice work by the way.
Cheers Kerrin
|
|
|
|
Post by ettingtonliam on Apr 17, 2020 2:40:43 GMT
David Piddington's original design for the Locomotion boiler had a couple of steel items silver soldered to the boiler shell, but was quickly redesigned to eliminate this after it was discovered that citric acid rotted the steel away, so be careful when pickling galvanised steel not to leave it in too long.
|
|
|
|
Post by springcrocus on Apr 17, 2020 6:57:40 GMT
Kerrin: there is a slight patina but that mostly wipes off. After a few minutes in the pickle, effervescence starts and gets quite prolific within ten minutes. The normally clear solution goes cloudy and the reaction gives off hydrogen gas. When the liquid goes clear again the reaction is finished and the zinc is gone. There is a black precipitate that sinks to the bottom which I assume is an insoluble zinc compound but could be displaced copper. My pickle is a pleasant blue colour which comes from the dissolved copper gained during the boiler build. I hope it doesn't get exhausted before I have finished the loco bodywork. It takes ages in fresh pickle.
Richard: yes, steel will dissolve in citric acid but at a far slower rate than zinc or copper compounds. I have left a handful of rusty 6BA bolts in the pickle for 48 hours and they clean up well with hardly any material loss. However, last year I noticed some discolouraton around the rivet heads in some riveted steel that I had pickled. I now scrupulously wash any pickled steel items in hot, soapy water afterwards.
Regards, Steve
|
|
|
|
Post by 92220 on Apr 17, 2020 8:33:39 GMT
Hi Kerrin.
If you want a copper plating on mild steel without electrolysis , you can use a copper sulphate solution with a small amount of sulphuric acid in it. When I was a trainee draughtsman, doing my workshop training, we used the copper sulphate solution to check whether a piece of metal was mild steel or stainless. The mild steel always came out copper coated and the stainless stayed self colour. You don't need much sulphuric to make it work. From what Steve has said, citric acid might also work, instead of sulphuric acid. The plating is not as resistant to rubbing off as electro-plated is though.
Hi Steve.
The citric acid trick is handy to know. I use a dilute phosphoric acid bath for most of my mild steel parts, . It coats the steel with a coating of iron phosphate which kills rust and also prevents it coming back. It also provides an ideal surface for painting. If anyone wants to use phosphoric acid, it is available on ebay quite cheap. It also needs to be diluted to around 15% acid to water. I'm not sure of the chemistry, but diluting the acid makes it work much stronger on rust. A 10 minute soak will usually clean up most rusty parts. Actually, if I want the phosphate coating to REALLY stick, I sand blast the steel part and then ALLOW it to rust, then pop it in the phosphoric. The converted rust helps the phosphate to adhere well to the surface, and not rub off,because, like the rust, it becomes almost part of the metal surface crystals.
Bob.
|
|
|
|
Post by coniston on Apr 17, 2020 9:29:09 GMT
I'm definitely going to have to try the Citric acid solution for both de-rusting and removing zinc, thanks for the suggestion Steve, It sounds like it is worth preparing it by leaving a bit of copper in there for some time before it becomes more useful on steel then. Currently I have been using Hydrochloric acid for de-rusting only because I 'acquired' some a couple of years ago along with a supply of sulphuric acid It is used in the steel industry for pickling steel after the hot rolling process. It is really nasty stuff and I dilute it 10% with water (Adding acid to the water). And I only use it and keep it outside, it really does get rid of rust.
Chris D
|
|
|
|
Post by steamer5 on Apr 17, 2020 9:36:21 GMT
Hi Chris,
Wonder if dissolving some copper sulphate in the citric acid would work?
Looks like, once we get out of lock down, a trip to the home brew suppler....NOW those guys should be an essential service, .....who also happen to be a tool suppler...best of both worlds, to get citric acid, then the garden center for some copper sulphate & give it a try.
Cheers Kerrin
|
|
|
|
Post by chester on Apr 17, 2020 17:25:42 GMT
Hi Kerrin in the uk food quality citric acid on ebay is quite cheap diy brew shops are expensive i bought it for about £5 a kilo two years ago.
|
|
|
|
Post by Jim on Apr 18, 2020 4:04:39 GMT
I'm definitely going to have to try the Citric acid solution for both de-rusting (snipped) Chris D Another alternative 'de ruster' is cold black tea. A chap I knew ran an antiques business and used a bowl of cold black tea as a receptacle for placing rusty steel antique items. I've tried it and over night the tannin in the tea reacts with the rust turning the rust to a black sludge that is easily rinsed off with the aid of a brush. These are the Britannia's tender wheels which came up very nicely after an overnight soaking in the black tea. The wheels had been stored in a tin that had collected water.
Jim
|
|
|
|
Post by steamer5 on Apr 18, 2020 7:32:41 GMT
Whoops!
Sorry Steve for sending your thread down a side track! Still some interesting stuff all the same.
Bob,
If you dissolve some zinc in the phosphoric acid, you’ll get a zinc phosphate coating. I’m struggling to remember the ins & outs of why dilute acid is more aggressive, it’s been a year or three, I’m sure somebody here will know the answer!
Chester, thanks for the tip.
Jim,
Well that’s a novel use for tea! Steel boiler water treatment, as supplied for our sizes uses tannin based treatment, but I guess you already know this!
Cheers Kerrin
|
|
|
|
Post by 92220 on Apr 18, 2020 10:17:04 GMT
Hi Kerrin.
I wasn't sure of the reason for diluted acid being more aggressive, so googled it. Here is the answer:- This promotes attack by the acid. ... In case of weaker acids, the dissociation increases with dilution, which means that as more and more water is added, more hydrogen ions will be generated. By and large, dilute sulfuric acid is more harmful than concentrated sulfuric acid.
Bob.
|
|
|
|
Post by Jim on Apr 18, 2020 10:55:35 GMT
You're right Kerrin I use the commersial tannin water treatment in the Burrell's steel Briggs Boiler.
Jim.
|
|
|
|
Post by steamer5 on Apr 18, 2020 22:20:08 GMT
Hi Bob,
Thanks for the info! Many years ago I worked in industrial labs but time tends to remove info like this!
On the sulphuric acid side of things, I worked in a fertilizer manufacturing plant lab as my first job out of school ( it was also my first introduction to things to do with boilers & steam!) I remember being told of a guy who, for what ever reason, stepped on to a tarpaulin cover that was covering a hole which had the mixer remove, he feel into the vessel which still had acid in it, not a lot of level, the acid was concentrated, >98% in strength, the guy had his boot filled with it. He was fine UNTIL the stuck a hose into his boot to wash the acid out! In case you guys have never added acid to water, PLEASE NEVER EVER BE TEMPTED TO ADD WATER TO ACID! The resultant mixture gets to almost boiling in the initial stages of the addition, best to add a little, wait for it to cool some, add more , repeat.
Cheers Kerrin
|
|
|
|
Post by d304 on Apr 18, 2020 23:20:26 GMT
Morning everyone
White vinegar is 10% citric acid. I bought some at your local shop Jim, for $2.00 for two litres. I needed it at the time to de-rust a full set of wheels for a 71/4 A3 A4 wheels that had been left on a barn floor for twenty years. I also use the citric acid for cleaning brass and copper prior to braising.
As for the disposal of the acid it becomes my weed killer.
Regards
David
|
|
|
|
Post by springcrocus on Apr 23, 2020 13:33:52 GMT
I'm not modelling the handbrake system as drawn, it relies on the tender chassis and it's tank being permanently joined. I do, however, want a handbrake and have knocked up my own version. It relies on a strong spring to hold the brakes on when in the steaming bay, for instance, but a cam operates against a lever to free the brakes for normal operation. I haven't bothered to describe this in detail because it will probably be of very limited interest. I also wanted a means of clipping the extra water pipes to the chassis and the first picture shows milled cutaways for the 1/4" pipe. These are for the handpump and the axle pump return.  A little gearbox was made to move the operating point to a location clear of the wheels. The gears came from the "useful one-day" box. 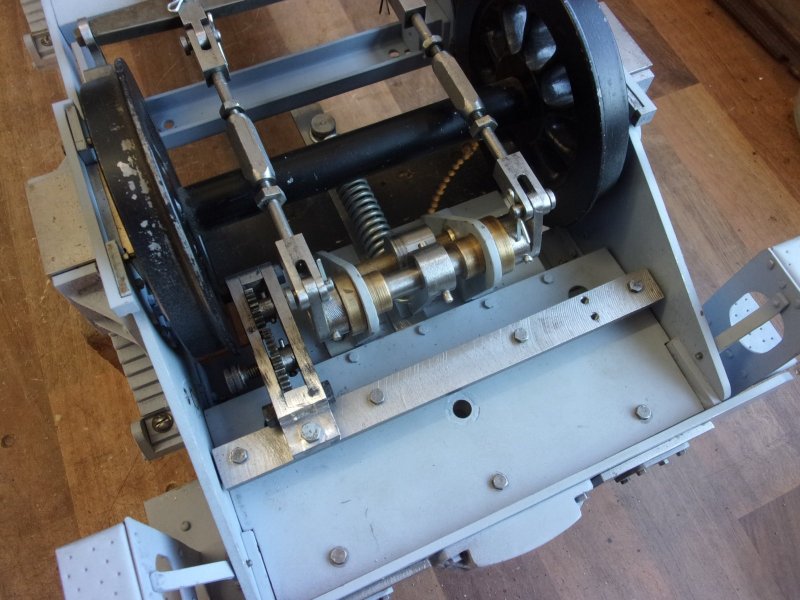 This view shows the cam with it's locking flat, the position that holds the brakes off. The spring is an old bed-frame spring. 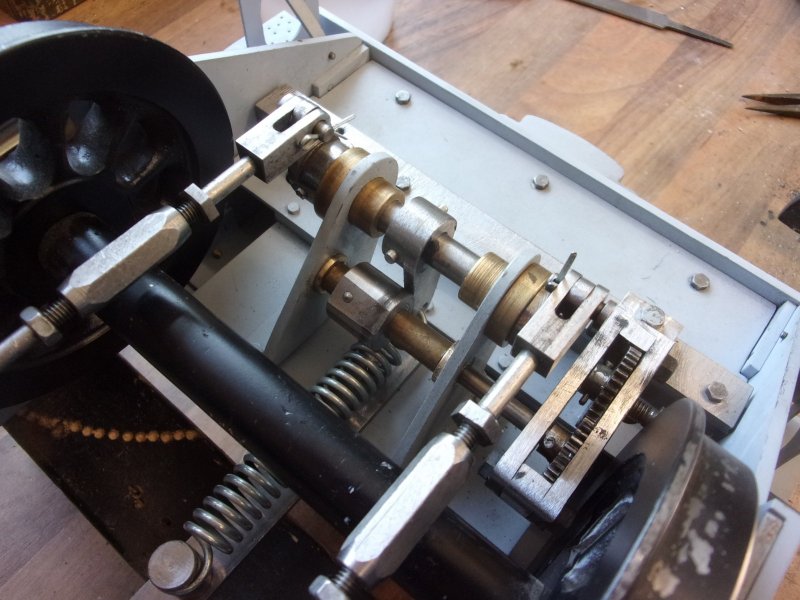 The final view shows the thing assembled with the pipes in place and a T-bar Allen key poking through a hole in the side of the tender chassis. 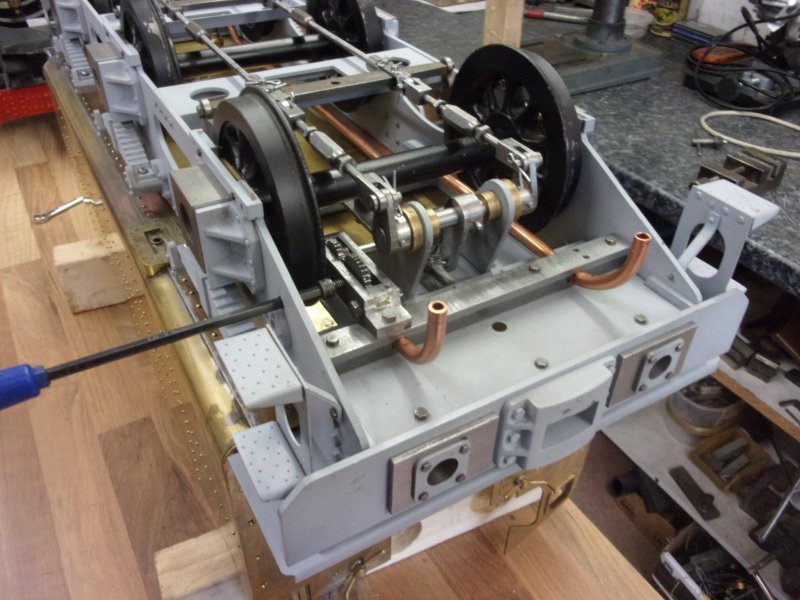 Once the turnbuckles were adjusted up, it works well and does just what I want it to but it won't suit most people. Steve |
|
|
|
Post by coniston on Apr 23, 2020 21:24:35 GMT
Steve, it may not appeal to every one but it's a neat solution as an alternative and serves your purpose very well. It solves a problem I've seen of damage to the traditional screw mechanism by lack of care when placing the tender on the rails or in the steaming bay. I've seen tenders come off in the steaming bay when the loco is pushed backwards and either not aligned to the track or by a bit of over enthusiastic pushing.
Chris D
|
|
|
|
Post by Jim on Apr 23, 2020 23:23:56 GMT
As Chris says it's a very neat solution Steve. I must admit I have opted for the use of 2 red flagged sprags to lock the drivers and the tender wheels while setting up in the steaming bay or mucking about at home and even then I still have a G clamp clamped to the track in front of the loco as aback up. There is no more sickening sound than that of a loco or tender rolling off the end of the steaming track.
Jim
|
|
|
|
Post by steamer5 on Apr 24, 2020 7:52:15 GMT
Hi Jim,
How about an engine trying to jump the gap of a part open turn table & sliding on its side, on concrete, to get wedged between the elevated track & the step up to a concrete path?
No it wasn’t me! Happened before a joined the club........sends a shiver down the spine! A bit like the sticking CRUNCH as a loco rolls off the steaming bay!
Steve,
Nice work, she’s coming on very nicely!
Cheers Kerrin
|
|
|
|
Post by Roger on Apr 24, 2020 8:50:39 GMT
Hi Jim, How about an engine trying to jump the gap of a part open turn table & sliding on its side, on concrete, to get wedged between the elevated track & the step up to a concrete path? No it wasn’t me! Happened before a joined the club........sends a shiver down the spine! A bit like the sticking CRUNCH as a loco rolls off the steaming bay! Steve, Nice work, she’s coming on very nicely! Cheers Kerrin Very neat Steve. Kerrin, I've always wondered why clubs don't all have stops or chocks on the steaming bays. Everyone seems perfectly happy to have their locomotives where they could just roll off the end. I don't understand it. I'll be making chocks, the thought of it running off the end is horrendous and easily avoidable. |
|